ARTÍCULOS ORIGINALES
Particulate bone matrix usage for alveolar
bone conservation. a histomorphometric study.
Sebastián Fontana1, Luis Plavnik1-2,
Miguel Filippetti3, Alicia Inés Malberti1
Revista Facultad de Ciencias Medicas 2013; 70(3):115-122
1 - Chair of Histology,
Dentistry Faculty, National University of Córdoba,
Argentina.
2 - Science and Technology Area, CREO Foundation, Córdoba,
Argentina.
3 - Human Tissue Processing Plant, UNC-Biotecnia, National
University of Córdoba, Argentina.
Correspondence:
Dra Alicia Malberti
Cátedra de Histología, Departamento de Biología Oral,
Facultad de Odontología.
Universidad Nacional de Córdoba (UNC). Haya de la Torre s/n,
Ciudad Universitaria CP: 5000. Córdoba, Argentina. e-mail:
inesmalberti@gmail.com
This project was funded by a
subsidy from the Secretariat of Science and Technology at
Córdoba National University (SeCyT, UNC. Res 162/12).
Introduction
In dental practice, knowledge of bone tissue
is of fundamental importance because the practitioner must
deal on a daily basis with injuries and treatments that
directly or indirectly affect the maxillary bones. Such
dental processes as abscesses, cysts, tumours, trauma or
simply the inevitable post-extraction atrophy can cause
severe bone loss, with great decrease of the vertical
dimension.
After tooth extraction, the healing of the
alveolar bone includes clot formation and maturation, and,
at the final repair, alternated phenomena of bone apposition
and resorption take place 1-3. In general, it is
suggested that the ridges of the alveoli collapse and bone
volume decreases in the late stages of alveolar healing
4-5.
In an attempt to preserve the height and
volume of alveolar ridges, recent research has focused on
different filling materials and the reactions that they
promote inside tissues. Biomaterial-induced bone repair
starts with the proliferation of capillaries (angiogenesis)
and mesenchymal cell proliferation, which run between
interparticle spaces. Differentiated cells of the
osteoblastic lineage invade the area and usually attach to
the particle surface for osteoid matrix secretion that later
mineralizes 3,4.
Some of the options for bone fillings
6-9 are: a) autologous or autogenous grafts, (e.g.
autologous bone); b) homologous or allogeneic grafts or
allografts, (e.g. freeze dried bone allograft (FDBA) and
demineralized freeze-dried bone allograft (DFDBA), also
called demineralized bone matrix); c) heterologous grafts or
xenografts, (e.g. deproteinized bovine bone mineral - DBBM,
Bio-Oss®, Giestlich Pharma); and d) alloplastic or synthetic
grafts, e.g. bioactive glass and tricalcium phosphate. All
these materials, can mediate new bone formation by one/some
of these processes: osteogenesis, osteoconduction and/or
osteoinduction 6, 7,10,11.
In the references, the autologous bone graft
is generally considered as the gold standard 12-14
because it retains both cell vitality and bioactive
molecules, such as bone morphogenetic protein (BMP). Also,
auto-grafts revascularize easily and do not transmit
diseases. However, obtaining it requires a second surgical
procedure at a donor site, with the consequent risk of
postoperative complications 15,16. For this
reason, the use of substitute materials from human bone
banks has increased, and those most often used in the clinic
are FDBA and DFDBA 8, 16-18. In this sense, our
work group recently conducted a study on the repair of
alveoli at 30 days post-extraction 19 using
allograft particles, the bone matrix developed at Córdoba
National University (MO-UNC). We concluded that the
presence of these particles did not interfere with the
habitual repair of the post-extraction alveolus. During the
study period, the MO-UNC integrated compatibly with the
newly formed bone. It was also established that particle
shapes and dimensions (535.42 µm) were appropriate and
within the parameters described in other studies, which
suggest that the best osteogenic effects occur with specific
sizes ranging from 125 to 1000
mm
17, 20.
Having demonstrated that MO-UNC acts as a
biocompatible and osteoconductive material, we think that
this material could accelerate bone formation and favour the
preservation of the volume of post-extraction alveolar bone.
In this sense, the aim of the present study
was to evaluate morphologically and histomorphometrically
the effect of the bone matrix (MO-UNC) in the process of
post-extraction alveolar repair at different experimental
time points.
Material and Methods
Filling material features
The bone matrix developed at Córdoba National
University, is human bone tissue for therapeutic use from
the Bone Bank at Córdoba Hospital, authorized by the Unique
National Coordinating Central Institute of Ablation and
Implant (Instituto Nacional Central Único Coordinador de
Ablación e Implante, INCUCAI) from Argentina. In the
Human Tissue Processing Plant, cortical portion of the long
bones from cadaveric donors is selected. The bone is
processed in aseptic areas, lyophilized, ground and
sterilized by gamma radiation. The final bone particles
(Figure 1)
has been authorized by Argentina’s National
Medication, Food and Medical Technology Administration (Administración
Nacional de Medicamentos Alimentos y Tecnología Médica,
ANMAT) and registered as medical product (number 1007/1-2)
Surgical Procedure
Forty male Wistar rats weighing 80 g
(± 10, body weight) were used and kept in biotery. Rats
received a balanced diet and water ad-libitum. In all
cases, strict controls were carried out to reduce any pain
or discomfort in the laboratory animals, complying with the
standards of the National Institute of Health (NIH
Publication No. 8523 rev 1985). The experimental work
protocol was approved by the Committee of Research Bioethics
of the Faculty of Medical Science at Córdoba National
University.
The animals were anesthetized with Ketamine
solution (8 mg/100 g body weight; Ketamine Zoovet®,
Lab. Zoovet, Argentina) and Xylazine (1.28 mg/100 g body
weight; Sedomín®, Lab König SA, Argentina).
Molar
extraction procedures in the rat were carried out following
the methodology described by Guglielmotti and Cabrini 1.
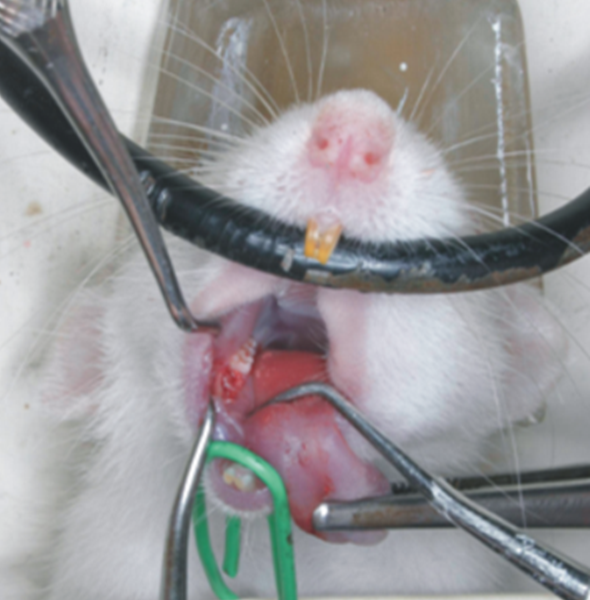
A special examination table was designed to immobilize the
animals and both first molars were extracted (Figure 2).
After extraction, the right alveoli were filled with 0.2 cm3
of MO-UNC particles (Experimental Group, EG), while
the left alveoli were left unfilled (Control Group, CG).
The animals were separated into four (4) groups (n=10 each
group) and, sacrificed at 0 h, 15, 30 and 60 days
post-extraction respectively. The hemi-maxillaries were
resected and fixed in a 10%, PH 7 formalin fixing solution
for 24 hours. All the samples were X-rayed, demineralized
and embedded in paraffin. Vestibulo-lingual cross sections
were made at the level of the mesial alveolus of the first
lower molar for microscopic study with hematoxylin-eosin
stain.
 |
 |
| Figure 1.
Microscopic appearance of MO-UNC particles.
(Original Magnification x 45). |
Figure 2.
Animal on the examination table with bonds keeping
the mouth open and overview of an empty first lower
molar alveolus. |
Analysis
A descriptive
analysis was made of the presence of MO-UNC particles in the
alveoli and the neo-formation of bone tissue in direct
relation to them (osseointegration), at the different time
points studied. Histomorphometric analyses were performed
using image analysis software (Image Pro-Plus 4.5). A
comparison of the total alveolar volume (TAV), height of the
buccal plate (Bh), height of the lingual plate (Lh) and
percentage of osseointegration (OI) of particles, was made
between EG and CG at each experimental time point. For the
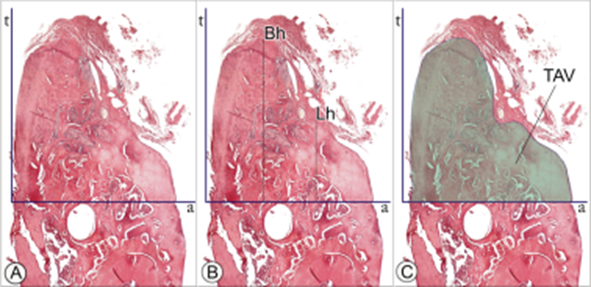
measurements (Figure 3), a tangent line was drawn to the
most salient point of the buccal plate (line t) and
another line perpendicular to t, passing through the
upper edge of the lower
dental nerve canal (line a). The whole
bone tissue with its bone marrow spaces, located above line
a, was considered as TAV. To define Bh and Lh, the
highest points in the buccal and lingual plates respectively
were marked. Lines were drawn from these points to the
intersection with line a, leaving lines B and L
demarcated. For the assessment of OI the perimeter of each
particle was marked and the amount of new bone in close
contact to its surface was measured. Data were statistically
analyzed by nonparametric ANOVA (Kruskall Wallis test),
setting p≤ 0.05 for statistically significant differences.
Results
Histological analysis of EG and CG, at each
experimental time point.
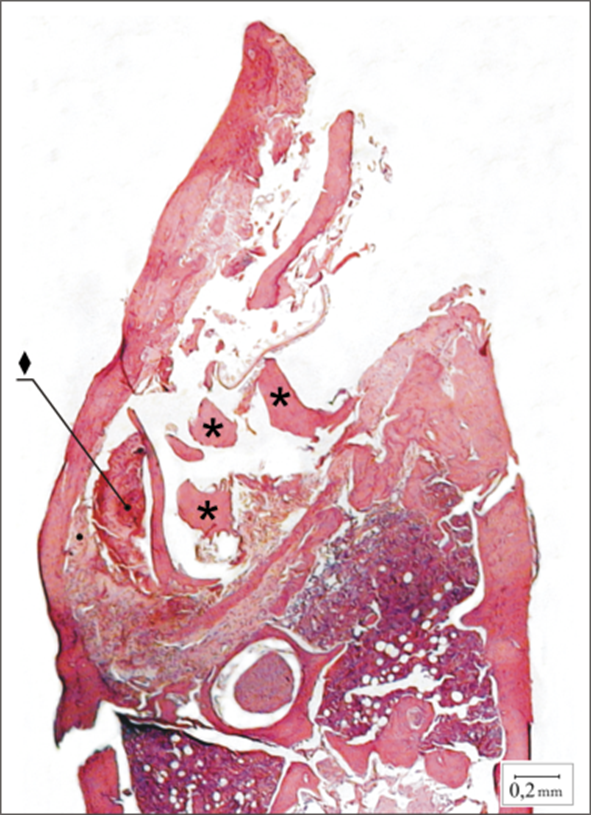
0 hours: in the
CG, the preparations showed the alveoli fully occupied by
clot and remnants of periodontal ligament. In the EG, bone
particles are arranged within the alveolus leaving wide
spaces between them and the alveolar bone
(Figure 4).
15 days: in the
controls,
newly-formed bone of the reticular type was observed in the
apical third of the alveoli. In the EG there was also
formation of reticular bone tissue and recently synthesized
bone was seen around the MO-UNC grafted particles.
30 days: in the CG, the alveoli were
completely occupied by lamellar, homogenous bone, similar to
the adjacent cortical bone. In the EG, reparative bone
tissue of the lamellar type was observed, as well as bone
matrix particles filling the alveolus. The newly-formed bone
had closely attached
to the surface of the particles: osseointegration (Figures 5
and 6).
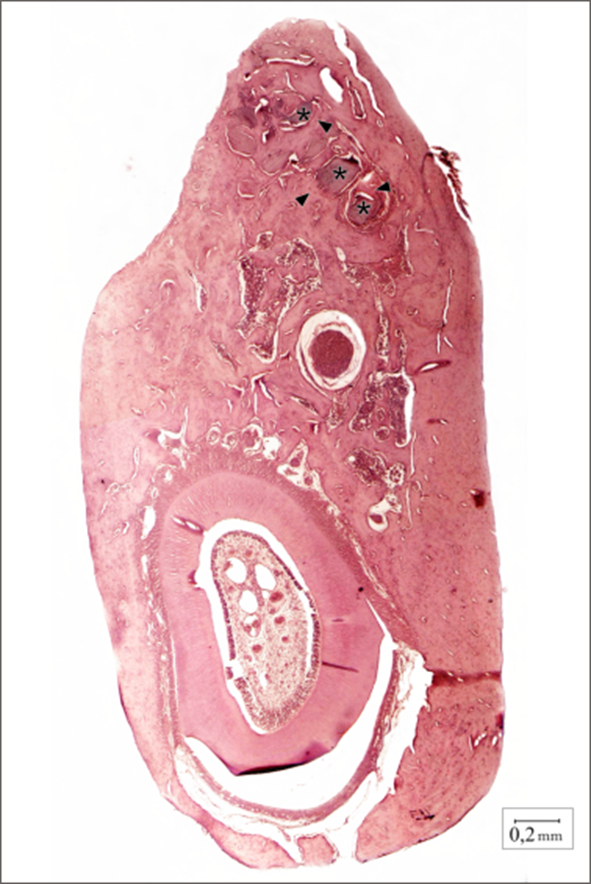
60 days: in the CG, the margins of the
alveolar ridge had definitively remodelled. In the EG, the
alveoli were occupied by particles completely surrounded by
newly-formed (osseointegrated) bone, of the lamellar type
(Figure 7). In
some
cases, the surface of the particles showed lacunae,
indicative of bone resorption.
Figures
|
 |
 |
 |
 |
 |
|
Figure 3. Lines
drawn over the slides to make the histomorphometric
measurements. A: lines t and a; B: buccal plate
height (Bh), lingual plate height (Lh) and C: total
alveolar volume (TAV). |
Figure 4.
Experimental
case at 0 h. Note the
clot (♦) and bone particles ()
inside the limits of the
alveolus (H/E Original
Magnification x 40). |
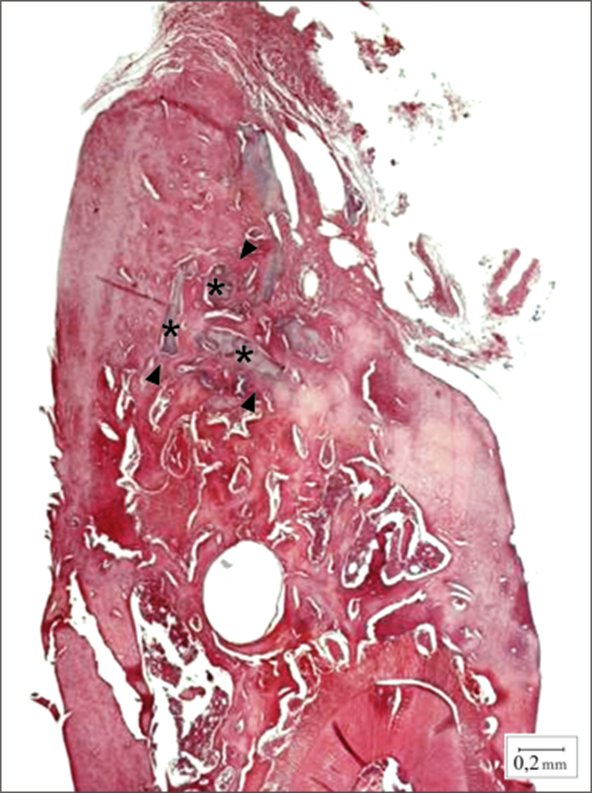
Figure 5. A complete mandible of EG, 30 days
post-surgery. Note the MO-UNC particles () filling
the alveolus and newly-synthesised bone (►), which
indicates the post-extraction repair (H/E Original
magnification x 40). |
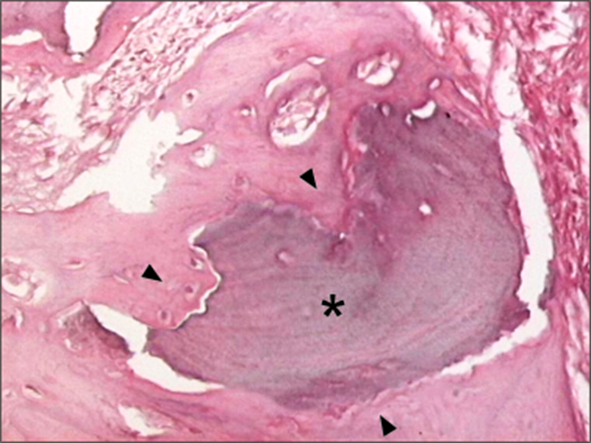
Figure 6. Mature lamellar bone (►) closely
attached to the surface of a particle () in EG, 30
days, showing osseointegration (H/E. Original
magnification x 400). |
Figure 7. A complete mandible of EG, 60 days
post-surgery. Particles inside the alveolus are
completely covered by lamellar bone. Alveolar ridge
has been completely remodelled (H/E Original
magnification x40). |
Histomorphometric analysis of alveolar ridges
The most significant histomorphometric values
are presented in Tables 1 and 2.
Bh and Lh: when comparing the heights of the
vestibular and lingual plates between the control and
experimental groups, no statistically significant
differences were found at any of the time points
studied.
TAV: At 15, 30 and at 60 days, the TAV values
were greater for EG than for the respective CG. However the
differences were statistically significant only at 60 days
post-surgery
(Table 1).
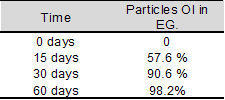
Percentage of ossointegration (OI): When
analyzing the cases treated with MO-UNC (EG), at the
different times of the study (15, 30 and 60 days),
statistical analysis showed that OI of the particles
increased as a function of
time (Table 2).
Tables
Table 1. TAV values
between CG and EG (expressed in mm2)
at the different experimental time points.
Note the statistically significant differences at 60 days
post-extraction.
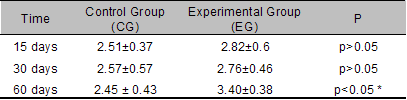
Table 2. Percentage of OI of the particles at
the different EG study time points. The increase in this
parameter is significant as a function of time.
Discussion:
This
experimental study
of post-extraction alveolar repair
aims to clarify some aspects about the biological effect of
a new allograft based on freeze-dried human bone,
the bone matrix developed
at Córdoba National University, (MO-UNC).
The first alternative to auto-grafts are the
substitutes from human tissue banks (allografts)
which have
mineral content (FDBA) or are demineralised (DFDBA). Another
alternative are xenografts and,
among them, the one which has been widely reported in the
scientific literature, is the bone of bovine origin (DBBM)
7-11. A recent literature review concluded that a lack
of available scientific data hampers to make clear
recommendations on the choice of a specific material for
bone regeneration 21.
This is probably due to controversies over
experimental methodologies and the interpretation of the
results 22. A comparative controlled study
suggests that some filling procedures may limit, but not
eliminate, the resorption of the alveolar ridge 23.
Our experimental work clearly shows that the
material used osseointegrated to the cicatricial bone of the
post-extraction alveolus at all the time points studied (0h,
15, 30 and 60 days). In no case did the particles implanted
interfere with the usual repair of the alveolus and,
moreover, the MO-UNC served as an appropriate physical
matrix for the apposition of new bone (osteoconduction).
These results match those of Urist 10 and
Glowacki 24, who argue that filling non-critical
bone defects with biomaterials demonstrates the
osteocompatibility and osteoconductivity of bone substitute
materials. Histomorphometric data of the present study show
no significant statistical differences in the heights of the
buccal and lingual plates when comparing the experimental
group with controls. At the 15, 30 and 60 days studied, the
total alveolar volume (TAV) was higher in the alveoli
treated with MO-UNC, compared with controls, but the
differences were statistically significant only at 60 days
post-extraction. This seems to indicate that the filling
used may promote bone modeling of the alveolus and prevent
the long-term contraction of the marginal ridge, which is
consistent with other studies in which bone fillings were
used alone or combined with osseointegrated implants
2,5,12,14,21,24. We also obtained histomorphometric
data of the percentage of osseointegration of the particles.
This was seen to increase as a function of the times studied
and, at 60 days (EG), reached more than 95% coverage of the
particles with newly-formed bone, values comparatively
higher than the ones reported by Carmagnola et al 18.
At
60 days post-surgery, we microscopically
observed lacunae and irregular areas on the surface of the
particles of MO-UNC in the experimental group. We infer that
this may be related to a phenomenon of resorption of the
material at this advanced stage of repair of the alveolus.
Thus, we suggest that the grafted material is biocompatible
because, as described previously, biomaterials must meet the
requirement of being degraded progressively within the
tissues 14.
Other studies 25, 26 reported
that placing certain type of filling particles produces a
delay in bone repair. This delay is attributed to a foreign
body reaction after implantation of the particles, which
were surrounded by multinucleated cells and inflammatory
tissue. In our study, there were neither inflammatory
phenomena nor delay in bone remodelling at any of the time
points studied.
The histomorphometric data of total alveolar
volume, comparing CG and EG, suggest that MO-UNC promotes
conservation of the alveolar ridge post-extraction. The
results obtained using this animal model might provide data
of interest about the behaviour of biomaterials in bone
tissue that can be applied in clinical practice. We believe
that more studies should be made to determine the course of
the particles over longer time periods.
Conclusions
In view of the results obtained in this study
and considering the limitations of the model used, we
suggest that:
Bone matrix-UNC (MO-UNC) does not interfere
with the process of repair of the post-extraction alveolus
and the particles osseointegrate to the newly-formed bone.
This type of filling prevents the collapse of
the post-extraction bone ridge.
The percentage of osseointegration of MO-UNC
particles in post-extraction alveoli increases as a function
of the time.
|
ACKNOWLEDGMENTS:
The authors wish to thank Dr. Mabel
Brunotto for her valuable contribution in the
statistical analysis of data. |
References
1-
Guglielmotti MB, Cabrini RL.
Alveolar wound healing and ridge remodeling after
tooth extraction in the rat: a histologic,
radiographic, and histometric study.
J Oral Maxillofac Surg. 1985;
43:359-64.
PubMed
2-
Araújo MG, Lindhe J.Dimensional
ridge alterations following tooth extraction. An
experimental study in the dog.
J Clin
Periodontol. 2005; 32:212-18.
PubMed
3-
Cardaropoli G, Araújo M, Lindhe J.
Dynamics of bone tissue formation in tooth
extraction sites. An experimental study in dogs.
J Clin Periodontol. 2003; 30:809-18.
PubMed
4-
Araújo M,
Linder E,
Wennström J,
Lindhe J.
The influence of Bio-Oss Collagen on healing of an
extraction socket: an experimental study in the dog.
Int J Periodontics Restorative Dent.
2008;28:123-35.
PubMed
5-
Heberer S, Al-Chawaf B, Hildebrand
D, Nelson JJ, Nelson K.
Histomorphometric analysis of extraction sockets
augmented with Bio-Oss Collagen after a 6-week
healing period: a prospective study.
Clin Oral Implants Res. 2008; 19:1219-25.
PubMed
6-
Cooper L. Biologic
Determinants of bone formation for
osseointegration: Clues for future clinical
improvements. J Prosthet Dent. 1998; 80:439-49.
PubMed
7-
Marx RE, Carlson ER, Eichstaedt RM,
et al.
Platelet-rich plasma. Growth factor
enhancement for bone graft. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 1998; 85:638-46.
PubMed
8-
Misch CE, Dietsch F. Bone grafting
materials in implant dentistry.
Implant Dent. 2003; 2:158-67.
PubMed
9-
Caneva M,
Botticelli D,
Morelli F,
Cesaretti G,
Beolchini M,
Lang NP.
Alveolar process preservation at implants installed
immediately into extraction sockets using
deproteinized bovine bone mineral. An experimental
study in dogs.
Clin Oral Implants Res.
2012; 23:789-96.
PubMed
10-
Urist MR. Bone:
formation by autoinduction. Clin Orthop Relat Res.
2002; 395:4-10.
11-
Fontana S, Olmedo D, Linares J,
Guglielmotti MB, Crosa ME.
Effect of Platelet Rich Plasma on the
Peri-implant Bone Response. An Experimental Study.
Implant Dent. 2004; 13:73-8.
PubMed
12-
Becker W, Becker BE, Caffesse R. A
comparison of demineralized freeze-dried bone and
autologous bone to induce bone formation in human
extraction sockets.
J Periodontol. 1994; 65:1128-33.
PubMed
13-
Schwartz Z, Mellonig JT, Carnes DL y
col. Ability of commercial demineralized
freeze-dried bone allograft to induce new bone
formation.
J Periodontol. 1996; 67: 918-926.
PubMed
14-
Chiapasco M, Casentini P, Zaniboni M.
Bone augmentation procedures in implant dentistry.
Int J Oral Maxillofac Implants. 2009; 24:237-59.
PubMed
15-
von Arx T, Häfliger J,
Chappuis V.
Neurosensory
disturbances following bone harvesting in the
symphysis: a prospective clinical study.
Clin Oral Implants Res. 2005 Aug; 16(4):432-9.
PubMed
16-
Zárate-Kalfópulos B, Reyes Sánchez A.
Injertos óseos en cirugía ortopédica.
Cir Ciruj. 2006; 74:217-22.
Full text
17-
Guglielmotti MB, Alonso ME, Itoiz ME,
Cabrini RL. Increased osteogenesis in alveolar wound
healing elicited by demineralised bone powder.
J Oral Maxillofac Surg. 1990;
48:487-90.
Abstract
18-
Carmagnola D, Adriaens
P, Berglundh T.
Healing of human extraction sockets filled with
Bio-Oss.
Clin Oral Implants Res. 2003;14:137-43.
PubMed
19-
Fontana S, Plavnik LM, Renou SJ,
González de Crosa ME.
Bone substitute in the repair of the post-extraction
alveolus.
Acta Odontol Latinoam. 2010;23:42-6.
PubMed
20-
Shapoff CA, Bowers GM,
Levy B, Mellonig JT, Yukna RA. The effect of
particle size on the osteogenic activity of
composite grafts of allogeneic freeze-dried bone and
autogenous marrow. J Periodontol. 1980;5 1:625-30.
PubMed
21-
Piattelli A, Scarano A, Corigliano M,
Piatelli M. Comparison of regeneration with the use
of mineralized and demineralised freeze dried bone
allografts: a histological and histochemical study
in man. Biomaterials. 1996; 17:1127-1131.
PubMed
22-
Chiapasco M, Zaniboni
M, Boisco M.
Augmentation procedures for the rehabilitation of
deficient edentulous ridges with oral implants.
Clin Oral Implants Res. 2006;17:136-59.
PubMed
23-
Horváth A,
Mardas N,
Mezzomo LA,
Needleman IG,
Donos N.
Alveolar ridge preservation. A systematic review.
Clin Oral Investig.
2013; 17(2):341-63.
PubMed
24-
Glowacki J. A review of
osteoconductive testing method and sterilization
processes for demineralised bone. Cell and Tissue
Banking 2005; 63-12.
PubMed
25-
Araújo M, Linder E, Lindhe J.
Effect of a xenograft on early bone formation in
extraction sockets: an experimental study in dog.
Clin Oral Implants Res. 2009; 20:1-6.
PubMed
26-
Calixto RF,
Teófilo JM,
Brentegani LG,
Lamano-Carvalho TL.
Grafting of tooth extraction socket with inorganic
bovine bone or bioactive glass particles: comparative
histometric study in rats.
Implant Dent.
2007; 16(3):260-9.
PubMed
|