ARTICULO ORIGINAL
Gross Motor Function Classification System
in Patients with Mucopolysaccharidosis.
Sistema de Clasificaciòn de la función motora en pacientes
con Mucopolisacaridosis
Marcos Almeida Matos1, Rosa Barreto2,
Vitor Quadros1, Carlos Eduardo Penha1, Angelina Xavier
Acosta2.
Revista Facultad de Ciencias Medicas 2013; 70(4):201-206
1. Bahian School of Medicine
and Public Health, Salvador- Bahia, Brazil.
2. Faculty of Medicine of Bahia, Federal University of Bahia
and Medical Genetics Service (COM-HUPES/UFBA), Salvador-Bahia,
Brazil.
Corresponding author:
Marcos Almeida Matos
Rua da Ilha, 378, Casa 21, Itapuã
Salvador-Bahia, Brazil
CEP 41620-620
e-mail:
malmeidamatos@ig.com.br
Disclosure of financial interest and Conflict of interest:
All authors have none to declare.
1. Introduction
Mucopolysaccharidosis (MPS) are a group of rare
orthopedic disorders caused by heterogeneous genetic
abnormalities in which lysosomal storage alteration lead to
intracellular accumulation of glycosaminoglycans (GAGs) that
injure and create dysfunction of varying degrees in multiple
organs and systems, in a progressive and lethal way1. The
type of MPS can be classified according to the enzyme defect
by which it is determined or according to its clinical
manifestations and the progression of the disease1,2,3
The impairment of the musculoskeletal system or “dysostosis
multiplex” is a common feature in all MPSs1,2. It is
characterized by osteoarticular deformities (kyphosis,
scoliosis, knee valgus, equinovarus), joint stiffness with a
loss in range of motion (ROM), and upper motor neuron
impairment (myelopathy, hypertonia, spasticity)1,2.
There is no specific motor function classification system
for individuals with MPS. However, the progression of motor
function impairment such as walking, sitting, and functional
independence in day to day activities is directly related to
the severity of the disorder1,2,3.
The “Gross Motor Function Classification System” (GMFCS) was
developed to describe the severity of motor function
impairment in patients with cerebral palsy4. This scale is
stable for patients between the ages of 2 and 12, and its
validity and reliability have given it international
acceptance and use4,5. Such as in cerebral palsy, patients
with MPS show motor function impairment that is associated
with progressive joint stiffness and upper motor neuron
injury.
The GMFCS could also be used as a scale for systematic
grading of MPS. This will allow for normalization of the
functional motor severity in these subjects, enabling data
homogenization and the comparison of treatment outcomes in
patients with similar disorders. The objective of this study
is to verify that the GMFCS scale can be used to evaluate
motor function impairment in patients with MPS.
2. Material and Methods
This is a retrospective study, based data from the
medical records of 22 patients with different types of MPS
who were on enzyme replacement therapy (ERT) in the
pediatric wing of the Hospital. The patient data were
evaluated during ERT. This study was in accordance with the
ethical standards laid down in the 1964 Declaration of
Helsinki; informed consent was obtained for all patients and
the protocol was approved by the institutional Ethics and
Research Committee.
All treated patients were clinically evaluated using a
standardized form for musculoskeletal system evaluation that
had been previously prepared by the research team. The form
contained general information (socio-geographic and clinical
data) as well as specific data collected from locomotor
examinations. All patients were evaluated by at least two of
the authors together at the time of ERT.
Specific data collected on the form were: an assessment of
global motor function; measured range of motion of the knee,
elbow, and shoulder joints; evaluation of the main deep
tendon reflexes (brachioradialis, triceps, biceps, patellar,
and Achilles’); and an evaluation of hand function. Global
motor function was measured using a score from the GMFCS
(Global Motor Function Classification System), which is an
evaluation system that was initially created for patients
with cerebral palsy4. The joints’ ranges of motion were
measured using a simple universal goniometer6. Reflexes were
evaluated in the conventional way, and the Wexler Scale7 for
their quantification. Hand function was graded using the
scale proposed by Haddad et al.8.
GMFCS was assessed at two different times. In the first time
patients were classified by three observers, named A, B and
C: the right classification was obtained by consensus. The
second evaluation was performed one week later and the
patients were evaluated by observer A only. We assumed that
one week was insufficient to produce any significant
clinical alterations. Data from the evaluations were used to
assess reliability; inter-observer agreement (between A, B
and C) and intra-observer agreement (between A at the
beginning and A one week later).
For research purposes, the data were collected and divided
into two groups. The first group, “GMFCS=1”, was composed of
patients who were assessed to be at Level 1 on the GMFCS (with
the least amount of impairment). The second, “GMFCS>1”,
included patients who belonged to the other levels (two
through five) on the GMFCS. This dichotomous division of the
scale that originally has five stages was used to increase
statistical analysis power, avoiding a study with small
numbers in each group.
Reliability was assessed by means of kappa statistics
according to Fleiss (1981)9 and the degree of concordance
was according to Landis and Koch (1977)10. The data from
both groups were presented in descriptive tables and a
comparison between them was done, looking at significant
differences that would validate the GMFCS scale as an
effective marker of motor function in patients with MPS.
Discrete variables were compared using the chi-square test,
while continuous variables were compared using the Student’s
t-test. Correlation studies were also performed (Pearson or
Cramer) between variables considered statistically or
clinically significant. In all the hypothesis testing,
p<0.05 was used as the level of statistical significance.
3. Results
14 Patients were classified as GMFCS1, 3 were GMFCS2, 1
was GMFCS3, 1 were GMFCS4 and 3 were GMFCS5. Inter-observer
agreement was 0,89 (confidence interval = 78-96) and intra-observer
agreement was 0,93 (confidence interval = 84-99) Both
results were considered almost perfect according to Landis
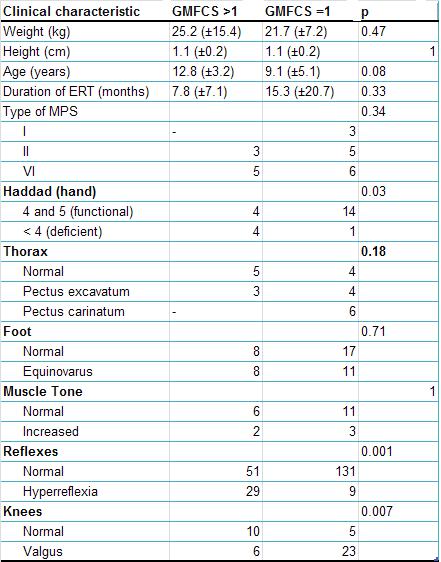
and Koch10. Table 1 shows the main characteristics of
patients, distributed according to the severity of their
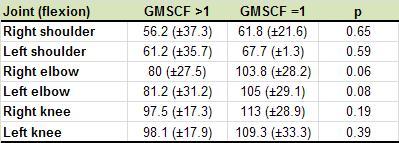
motor impairment in accordance with the GMFCS scale. Table 2
demonstrates the ranges of motion (flexion) of the shoulder,
elbow, and knee joints, also distributed according their
severity on the GMFCS.
The median age of the patients in the study was 10.5 (±4.8)
years. Thoracic deformity was seen in 59% of the subjects;
31.8% had pectus carinatum and 27.2% had pectus excavatum.
The equinovarus deformity was found in 43.2% of examined
feet. Knee valgus was seen in 65.9% of the subjects.
Hyperreflexia was found in 17.3% of tested reflexes, while
27.2% of patients showed increased muscular tone. All
evaluated patients showed loss of range of motion to some
degree in the assessed joints (joint stiffness).
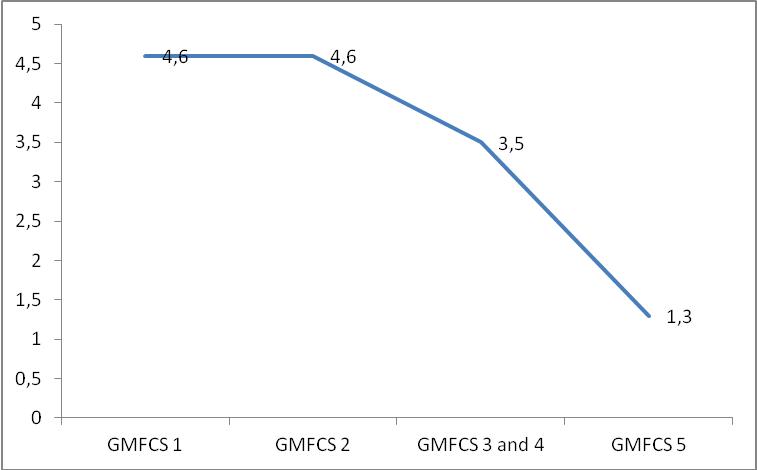
A notable association was found between the GMFCS and both
Haddad’s hand function scale and ROM of the elbows. For this
reason, tests were done to verify the existence of a
correlation between the GMFCS and Haddad’s scale, as well as
between the GMFCS and ROM of both the right and the left
elbow. The correlation with the flexion of the right elbow
was r=0.34 and with the left elbow was r=0.17; neither was
considered statistically significant. The correlation with
Haddad’s scale, however, was r=0.94, and statistically
significant (Figure 1).
To check the association between GMFCS and hyperreflexia,
Cramer’s contingency coefficient was used, which is equal to
the correlation coefficient of variables subjected to the
chi-square test. In this case, Cramer’s coefficient
regarding this association was 0.41 for association, showing
a p=0.001.
|
 |
 |
 |
| Tabla I |
Tabla II |
Figura I |
4. Discussion
Patients in this study had a high prevalence of
deformities in the thorax (59%), foot (43.2%), and knee
(65.9%) regions. Additionally, there was a high frequency of
signs of upper motor neuron involvement, evidenced by the
amount of hyperreflexia (17.3%) and hypertonia (27.2%).
These findings are characteristic of all MPSs, which to a
greater or lesser degree always come with skeletal
deformities such as genu valgum,equinus varus, pectus
carinatum and excavatum, and joint stiffness and
contractures1,2,3. Communicating hypertensive hydrocephalus,
while more common in MPS type I, can also occur in types II
and VI2,3. Cervical cord compression and myelopathy are also
often found in MPS. Their relation to hydrocephalus could
explain the increased presence of signs characteristic of
upper motor neuron impairment in the studied group. These
clinical characteristics confirm a clinical similarity
between MPS and cerebral palsy in relation to orthopedic
deformities and associated motor impairment.
GMFCS has been a simple and efficient method for classifying
MPS patients as well as it has showed an excellent intra and
inter-rate reliability. This is in accordance to the same
reliability evaluation obtained in a group of patients with
cerebral palsy used to validate GMFCS in Brazilian
patients11. The total of 22 subjects was insufficient to
divide them into multiple groups according to their severity
as scaled on the GMFCS, but the comparison of the less
impaired group to the group with more severely impaired
motor functions (all together) has shown that the GMFCS can
also be effective in detecting the severity of motor
function disability in patients with MPS.
The patients from both groups were considered homogeneous
for comparison purposes, with no differences in weight,
height, or time spent undergoing enzyme replacement. Despite
the absence of type I patients in the group “GMFCS>1”, there
was no significant difference with regards to the type of
MPS in each group (Table 1).
There was a greater incidence of deformity in the thorax and
knee regions among patients from the group “GMFCS=1”. The
majority of the deformities, however, were found in patients
with MPS types VI and I. Out of the subjects with type VI,
72.7% were found to have thoracic abnormalities and 100% of
individuals with type I had the same deformity. The fact
that there were no type I patients, and a lower frequency of
type VI patients in “GMFCS>1” could have contributed to the
small number of deformities in this group. The greater
prevalence of valgus deformities in the less severely
impaired group (GMFCS=1) could have been influenced by the
low age of these patients, taking into consideration that
this deformity tends to diminish between 4 and 12 years of
age12.
Hand function was significantly more impaired in the group “GMFCS>1”.
This reinforces the idea that this group has inferior
musculoskeletal function to the group “GMFCS=1”. The
functions that can be accomplished with an upper limb, such
as holding onto objects and dressing, grooming, and cleaning
oneself, are determinants in the activities of daily living
(ADLs) and quality of life of patients with MPS13. The
correlation between functional capacity as measured by the
GMFCS and hand function in patients with MPS (r=0.94,
p<0.05) indicates that the GMFCS also has the capacity to
stratify motor difficulties in performing ADLs and tasks
that involve the use of upper limbs.
Range of movement of the shoulder, elbow, and knee joints
was consistently lower in the group “GMFCS>1”, and further
evaluation of the right and left elbows showed a tendency
toward statistical significance among the comparisons
between the groups (p<0.1). It is possible that the GMFCS
could be an important indicator as to joint stiffness in
patients with MPS, however the sample size may have been
insufficient to prove this finding.
An important limitation of this study is the small sample
size, but MPS are a group of rare disorders and a series of
cases greater than 22 individuals is very difficult to come
by in literature. The goniometer as a measure of joint ROM
is a measure dependent on the examiner, however it is a
method with good validity and reliability6. In order to
minimize possible errors with the goniometer, we chose only
large movements (flexion), and their measurements were
considered only in the joints that are normally the most
impaired.
Another limitation that should be discussed is that the six-minute
walk test was not performed [14]. This test, however, has a
wide variability that makes it unsuitable for analysis
considering the fact that some of our patients were unable
to walk.
Our study is an original contribution to literature about
the possibility of using the GMFCS as an indicator for the
severity of musculoskeletal system impairment in patients
with MPS, and also demonstrate an excellent inter and intra-observer
agreement, much as it is in patients with cerebral palsy.
This scale correlated with hand function, upper motor neuron
involvement, and proved a reliable marker of joint stiffness
in this group of patients.
5. References
1. Neufeld EF, Muenzer J. The mucoplysaccharidoses. In:
Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler
KW, Vogelstein B (eds) The metabolic and molecular bases of
inherited disease, 8th ed. McGraw-Hill, New York, 1995;
p3421–3452.
2. Valayannopoulos V, Nicely H, Paul Harmatz P, Turbeville
S. Mucopolysaccharidosis VI. Orphanet J Rare Dis 2010; 5:5.
Full Text
3. Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L,
Guffon N, Meldgaard Lund A, Malm G, Van der Ploeg AT, Zeman
J. Mucopolysaccharidosis type II (Hunter syndrome): a
clinical review and recommendations for treatment in the era
of enzyme replacement therapy. Eur J Pediatr 2008;
167:267-77.
Full Text
4. Palisano R, Rosenbaum P, Walter S, Russel D, Wood E,
Galuppi B. Development and reliability of a system to
classify gross motor function in children with cerebral
palsy. Dev Med Child Neurol 1997; 39:214-23.
PubMed
5. Wood E, Rosenbaum P. The Gross Motor Function
Classification System for cerebral palsy: a study of
reliability and stability over time. Dev Med Child Neurol
2000; 42:292-296,
PubMed
6. Gajdosik RL, Bohannon RW. Clinical measurement of range
of motion. Review of goniometry emphasizing reliability and
validity. Phys Ther 1987; 67:1867-72.
PubMed
7. Cipriano JJ, Jahn WT. Photographyg manual of regional
orthopedic and neurological tests. 3th ed. Williams &
Wilkins, New York, 1999; p 57-69.
8. Haddad FS, Jones DHA, Vellodi A, Kane N, Pitt MC. Carpal
tunnel syndrome in the mucopolysaccharidoses and
mucopolilipidoses. J Bone Joint Surg 1997; 79-B:576-82.
9. Fleiss JL. Statistical Methods for Rates and Proportions,
2nd Edition, Wiley, New York 1981.
10. Landis JR, Koch GG. The measurement of observer
agreement for categorical data. Biometrics 1977; 33:
159–174.
PubMed
11. Hiratuka E, Matsukura TS, Pfeifer LI. Cross-cultural
adaptation of the gross motor function classification system
into Brazilian-portuguese (GMFCS). Rev Bras Fisioter 2010;
14:537-44.
PubMed
12. Salenius P, Vankka E. The development of the
tibiofemoral angle in children. J Bon Joint Surg Am 1975;
57A:259-61.
PubMed
13. Sifuentes M, Doroshow R, Hoft R, Mason G, Walot I,
Diament M, Okazaki S, Huff K, Cox GF, Swiedler SJ, Kakkis
ED. A follow-up study of MPS I patients treated with
laronidase enzyme replacement Therapy for 6 year. Mol Gen
Metab 2007; 90:171-80.
PubMed
14. Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM,
Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED,
Braakman T, Chadbourne E, Walton-Bowen K, Cox GF. Enzyme
replacement therapy for mucopolysaccharidosis I: a
randomized, double-blinded, placebo-controlled,
multinational study of recombinant human alpha-L-iduronidase
(laronidase). J Pediatr
PubMed
|