ARTICULO DE REVISIÓN
Disease Mongering in Neurological
Disorders
Silvia Kochen1-2 ; Marta Córdoba2
Revista Facultad de Ciencias Medicas 2013; 70(4):217-222
1-2 Centro de Neurociencias
Clínicas y Aplicadas. Epilepsia, Cognición y Conducta.
Sección de Epilepsia, Div Neurología, Hosp “R.Mejía” – Inst.
de Biología Celular y Neurociencias, Fac. Medicina, Univ.
Buenos Aires –Consejo Nacional de Investigación Científico y
Tecnológico (CONICET)
2 Div. Neurología. Hospital R. Mejía , Buenos Aires,
Argentina.
Correspondence should be
addressed to Silvia Kochen,
skochen@retina.ar
If out of curiosity, the readers take a few seconds to
search on the Internet the expression “diseases mongering”,
they will see that "to promote or sell disease" is an
enforced definition. They will also find out that the term
competes in popularity with many frequently used words, even
with popular actors or sportsmen. Besides it will appear a
number of “new diseases" or novel groupings or categories of
“old diseases”. The main and common characteristic of all
these "diseases" is that they are amenable to be treated
with drugs. The first reason seems to be the advances in
scientific knowledge. However, we should incorporate other
considerations such as the interest of the pharmaceutical
industry in selling their products.
Almost 20 years ago, Lynn Payer1 used the term “disease
mongering” for the first time as the strategy of the
pharmaceutical industry to expand the boundaries of
treatable illness in order to increase the market (Table 1)
2. This concept was recently defined as “the selling of
sickness that widens the boundaries of illness and grows the
markets for those who sell and deliver treatments.”3
Therefore, the pharmaceutical industry redefines what is
normal and what is pathological modifying the concept of
disease. Much disease mongering relies on considering normal
biological or social variation as pathologies. It also is
based on the portrayal of disease risk factors as if it was
a pathological state in itself. The vicious circle is
completed when pharmaceuticals are used to treat risk
factors because“ anyone who takes medicines is by definition
a patient”4-6).
Disease mongering exploits the deepest atavistic fears of
suffering and death. In neurology added aspects are related
to cognitive function, or illness that cause great
disability, high mortality, or are surrounded by stigma.
The principal aim of this paper is generate awareness in
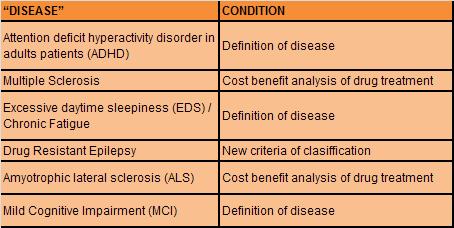
neurologists about of relatively new situation. We selected
some “new diseases” or disease mongering aspects in “old
disease” (Table 2). Although this corpus is just a sample,
it is useful to remark the effect of disease mongering in
neurology field. The choice was based on lack or weak
evidence in one or more condition: a- definition of disease;
or b- cost benefit analysis of drug treatment; or c- the use
of new classification that assign criteria of severity in a
disease. In every case, this situation implies the use of
expensive treatments. We describe a brief review of each of
the entities included.
|
 |
There is an important advertising campaign aimed at
potential consumers in order to “improve cognitive functions”
in mentally and neurologically healthy people, this
statement is very complexe and ambiguous. However, there is
a very large offer of drugs. The prescription of modafinil,
adrafinil, methylphenidate, inderal, piracetam, aniracetam,
amphetamines has increased in the last time7
Attention deficit hyperactivity disorder in adults
patients (ADHD)
National Institute for Health and Clinical Excellence (NICE,
2011) highlights the weak evidence in the definition of this
"disease": “this would be best conducted as an
epidemiological survey to answer the ADHD in adults”. In
1986, Nasrallah et al.8 reported brain atrophy in adult
males treated with amphetamines during childhood concluding:
“since all of the HK/MBD [hyperkinetic/ minimal brain
dysfunction] patients had been treated with psychostimulants,
cortical atrophy may be a long-term adverse effect of this
treatment”. In spite of this research other authors
published “Recent investigations with magnetic resonance
imaging (MRI) provide converging evidence that a refined
phenotype of ADHD is characterized by reduced size of the
frontal lobes and basal ganglia “9. However there had
been no such studies of ADHD-untreated cohorts10.
Although ADHD is a recognized pathology (ICD, DSM-IV) in
children, we can not stop thinking that the possibility of a
drug treatment can shoot some diagnostics, which does not
happen with disorders no treatable with drugs such as
dyslexia. ADHD is a symptom, a syndrome or a disease
diagnosed in 7.8% of U.S. children between 4-17 years (4.4
million children), according to results of a parent survey
conducted in 2003, of which 56% were medicated at the time.
Also in Spain the consumption of methylphenidate fivefold
from 1992 to 2001 with an estimated increase in annual
consumption of 8%. Could be possible to reduce diagnoses
understanding ADHD from a model of mental functioning rather
than from a model based on observable behavior and the sum
of symptoms, sometimes collected through global
questionnaires.5,11 In recent studies, in children and in
adults, observed increased in the prevalence of this
diagnosis with a trend of increasing prescribing of ADHD
drug treatment, however no demonstrate more evidence
diagnostic.12-14
Excessive daytime sleepiness (EDS) and Chronic Fatigue
Both categories EDS and Chronic Fatigue, can be
considered together taking into account that there is
overlaping on the definition used to define each of them,
even their existence as diseases or syndromes is contested
15. Nevertheless, there is abundant mass media advertising
refered to the “good results” achieved with psychostimulants.
A recently editorial of Neurology16, describe in relation
a paper published in the same journal17, as despite
having a Class I level of evidence in the treatment protocol
with modafinil in EDS and chronic fatigue, detailed analysis
showed did not improve fatigue symptoms, nor were there any
benefits in the psychomotor vigilance test18.
Mild cognitive impairment (MCI)
Despite there are clinical guides that contemplate mild
cognitive impairment as a defined disease19, their
consideration as a clinical entity according to some authors
is still a matter of debate. In this context of uncertainty,
clinical trials have been developed in the attempt to study
the effects of ChEIs (donepezil, rivastigmine, and
galantamine) in delaying the conversion from MCI to
Alzheimer disease or dementia.20 Although the use of
ChEIs in MCI was not associated thus far, with any delay in
the onset of AD or dementia, the safety profile showed that
the risks associated with ChEIs were not negligible21.
However appears information in scientific journals and in
mass media that encourages the use of these drugs to "prevent"
these dreaded diseases.
The disorders that mainly leads to a deterioration of motor
skill as multiple sclerosis or fatal disorder such as
amyotrophic lateral sclerosis, high influence for patients,
healthcare systems, and society as a whole:
Multiple Sclerosis
It is remarkable the profound analysis made by James
Raftery.22 What happened with multiple sclerosis risk
sharing scheme in United Kingdom represent a unique
situation where the NHS is paying for thousands of patients
to receive drugs that monitoring data suggest are not
effective. This scheme was set up in 2002 after the National
Institute for Health and Clinical Excellence (NICE)
recommended against the use of interferon beta and
glatiramer acetate. It is based on outcome analysis, not
only in cost benefit analysis. There was an agreement that
prices would be reduced if patient outcomes were worse than
predicted. Disease progression was not only worse than
predicted by the model used by NICE23, but even worse
than the untreated control group. In the same way, Cochrane
multiple sclerosis group has proposed that the efficacy of
interferon on exacerbations and disease progression in
patients with relapsing remitting MS was modest after one
and two years of treatment. Interferon administered by the
oral route was not effective for prevention of relapses.
Longer follow-up and more uniform reporting of clinical and
MRI outcomes among these trials might have allowed for a
more convincing conclusion.24,25 Recently in a
systematic review indicating that the anti-inflammatory
effect of Interferon β is unable to prevent MS progression
once it has become established.26 Nevertheless, there was
not any price reduction, moreover, in our country the prices
are more than double that in developed countries27.
Amyotrophic lateral sclerosis (ALS)
Several ALS therapies have shown promising results in
preclinical models of motor neuron disease. However, most of
them failed in human studies. A remarkable progress in
understanding the cellular mechanisms of motor neuron
degeneration has not been matched with the development of
therapeutic strategies to prevent disease progression or to
extend survival longer clinical trials.28
The current estimations of the cost-effectiveness of
riluzole must be analized cautiously. The uncertainty of the
benefits in the economic analysis is due to the
overconsideration of the survival gain that is experienced
in a determined disease stage. The quality of life utility
weights upon ALS health states and the gained life
expectancy for individuals who take riluzole. Estimates from
different trials suggest a gain in median tracheostomy free
survival time of 2 months to 4 months.29-31 This
treatment implies an increase in costs for the health
service. In addition to the unsatisfactory results, the
great impact of these costs in developing countries is
almost impossible to afford.
Drug resistant epilepsy
Epilepsy is one of the most prevalent neurological
disorders, that can be effectively prevented and treated at
an affordable cost for most of patients. Different
epidemiological studies estimated that up to 22.5% of
patients with epilepsy have drug-resistant epilepsy. In this
group the use of new drugs, more expensive, or nondrug
therapy such as epilepsy surgery should be considered.32
A new definition recently proposed for ILAE (International
League Against Epilepsy)33 includes in this category
patients that present seizures, opposed to patient seizure-
free, without special consideration, i.e. seizures without
consciousness, or only during sleep, or one seizure by year.
Although before to mentioned definition, there was no
unified definition of drug resistant epilepsy, those
patients who had affected their quality of life were
included as drug resistant or refractory epilepsy. Numerous
of patients now included in this group, were not consider in
this category previous to recent definition.34 It is
evident that in this new classification the concept quality
adjusted life year (cost/QALY) is not considered, and allows
that many more patients are liable to receive more expensive
and sophisticated treatments.
Conclusion
Pharmaceutical companies are not the only actors in this
field. Physicians, patients, mass media, politicians, also
play a role with different contributions, but together
multiply the effect of disease mongering. There must be
awareness of all stakeholders to know this problem at the
moment of making decisions related with diagnosis and
prescription.
It is necessary the implementation issues in latinoamerica
as the “Sunshine Act”, part of the Affordable Care Act,
created in USA, requires manufacturers to submit a list of
physicians and teaching hospitals who received from them a
transfer of value, but neither was implemented even in the
country of origin.
We consider it is essential that health professionals become
aware of this relatively new condition, which increases more
and more, probably have even more serious impact on
developing countries for their limited resources and
inequitable health condition.
References
1- Payer, Lynn “Disease-Mongers: How Doctors, Drug
Companies, and Insurers are Making You Feel Sick” Wiley,
USA, 1994.
2- Andresik D., " Disease Mongering: El arte de fabricar
enfermedades”, Biophronesis, Revista de Bioética y
Socioantropologia en Medicina, 2009, nº.2, v.4"
3- Jairaj Kumar C., Abhizith D., Ashwini K., Arunachalam K.,
Hegde B. M., “Awareness and Attitudes about Disease
Mongering among Medical and Pharmaceutical Students”, April
2006 , Volume 3 , Issue 4 ,e210, PLoS Medicine, published by
the US Public Library of Science (PLoS).
Full Text
4- Rose G (1985) Sick individuals and sick populations. Int
J Epidemiol 14: 32–38.
Full Text
5- Moynihan R. Cassels A. Selling sickness, Vancouver:
Greystone Books, 2005. ISBN 1553651316
6- Cuestas E. The new strategies for disease mongering; Rev
Fac Cien Med Univ Nac Cordoba. 2011;68(3):89-93.
Full Text
7- Gilbert D, Walley T, New B. Lifestyle medicines. BMJ.
2000; 321:1341
Full Text
8- Nasrallah HA, Loney J, Olson SC, McCalley-Whitters M,
Kramer J, et al. (1986) Cortical atrophy in young adults
with a history of hyperactivity in childhood. Psychiatry Res
17: 241–246
PubMed
9- Swanson J, Castellanos FX (1998) Biological bases of
attention deficit hyperactivity disorder. Program and
Abstracts, NIH Consensus Development Conference on Attention
Deficit Hyperactivity Disorder; 16–18 November 1998;
Bethesda, Maryland. pp. 37–42.
10- Baughman FA (1999) ADHD—Total, 100% fraud [video].
Produced from the official video of the NIH Consensus
Development Conference on Attention Deficit Hyperactivity
Disorder; 16–18 November 1998; Bethesda, Maryland.
11- Sixto ME., Martínez González C., Quintana Gómez JL.,
“Disease mongering, el lucrativo negocio de la promoción de
enfermedades”. Rev Pediatr Aten Primaria. 2009;11:491-512
Scielo
12- Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ,
Setyawan J, Erder MH, Neumann PJ. Economic impact of
childhood and adult attention-deficit/hyperactivity disorder
in the United States. J Am Acad Child Adolesc Psychiatry.
2012 Oct;51(10):990-1002.
PubMed
13- Barry CL, Martin A, Busch SH. ADHD medication use
following FDA risk warnings. J Ment Health Policy Econ. 2012
Sep;15(3):119-25.
PubMed
14- Batstra L, Frances A. DSM-5 further inflates attention
deficit hyperactivity disorder. J Nerv Ment Dis. 2012
Jun;200(6):486-8.
PubMed
15- Holgate ST, Komaroff AL, Mangan D, Wessely S. Chronic
fatigue syndrome: understanding a complex illness. Nat Rev
Neurosci. 2011 Jul 27;12(9):539-44.
Abstract
16- Richard M. Dasheiff, Editorial Neurology, Modafinil is
not the new caffeine Richard M. Dasheiff. Neurology
2010;75:1764–1765
Full Text
17- Kaiser PR, Valko PO, Werth E, et al. Modafinil
ameliorates excessive daytime sleepiness after traumatic
brain injury. Neurology 2010;75:1780 –1785.
PubMed
18- Kesselheim AS, Myers JA, Solomon DH, Winkelmayer WC,
Levin R, Avorn J. The prevalence and cost of unapproved uses
of top-selling orphan drugs. PLoS One. 2012;7(2):e31894ç
Full Text
19- The NICE guideline on supporting people with dementia
and their carers in health and social care this clinical
guideline has been amended to incorporate the updated nice
technology appraisal of drugs for alzheimer’s disease,
published in march 2011 (http://www.nice.org.uk/guidance/ta217 ).
20- Gauthier S., Touchon J., “Mild cognitive impairment is
not a clinical entity and should not be treated”. Arch
Neurol 62: 1164–1166. 2005
Abstract
21- Raschetti R., Albanese E., Vanacore N., Maggini M.,
“Cholinesterase inhibitors in mild cognitive impairment: A
systematic review of randomised trials”. PLoS Med 4(11):
e338. doi:10.1371/journal. pmed.0040338. 2007
Full Text
22- Raftery James. Multiple Sclerosis risk sharing scheme: a
costly failure. BMJ 2010; 340:c1672
Full Text
23- NICE. Multiple Sclerosis-beta interferon and glatiramer
acetate. Technology appraisal 32.2002
Full
24- G Rice, B Incorvaia, L Munari, G Ebers, et al.
Interferon in relapsing-remitting multiple sclerosis.
Editorial Group: Cochrane Multiple Sclerosis Group.
Published Online: 21 JAN 2009, The Cochrane Collaboration.
Published by John Wiley & Sons, Ltd.
25- Noyes K, Bajorska A, Chappel A, Schwid SR, Mehta LR,
Weinstock-Guttman B, Holloway RG, Dick AW.
Cost-effectiveness of disease-modifying therapy for multiple
sclerosis: a population-based study. Neurology. 2011 Jul
26;77(4):355-63.
PubMed
26- La Mantia L, Vacchi L, Rovaris M, Di Pietrantonj C,
Ebers G, Fredrikson S, Filippini G.J Interferon β for
secondary progressive multiple sclerosis: a systematic
review. Neurol Neurosurg Psychiatry. 2012 Sep 5. [Epub ahead
of print]
27- De Robles P., Recchia L., Gonorasky S. , Gonzalez
Aguilar P. El costo derivado del número necesario a tratar.
Revista Neurológica Argentina 2006; 31: 59-64
28- A Pratt, E Getzoff, J Perry, Amyotrophic lateral
sclerosis: update and new developments. Degener Neurol
Neuromuscul Dis. 2012 February; 2012(2): 1–14.
PubMed
29- Ludolph A. and Jesse S. “Evidence-based drug treatment
in amyotrophic lateral sclerosis and upcoming clinical
trials” Ther Adv Neurol Disord (2009) 2(5) 319–326
Full Text
30-
Guidance on the Use of Riluzole (Rilutek) for the
Treatment of Motor Neurone Disease Published by the National
Institute for Clinical Excellence January 2001
31- Miller RG, Mitchell JD, Moore DH. Riluzole for
amyotrophic lateral sclerosis (ALS)/motor neuron disease
(MND) Cochrane Database Syst Rev. 2012
PubMed
32- Shorvon SD. Epilepsia. 1996;37 Suppl 2:S1-S3. Review.
33- Kwan P., Arzimanoglou A., Berg A., Brodie M., Hauser W.,
Mathern G., Moshé S., Perucca E. , Wiebe S., French J.;
“Definition of drug resistant epilepsy: Consensus proposal
by the ad hoc Task Force of the ILAE Commission on
Therapeutic Strategies” ; Epilepsia Volume 51, Issue 6,
pages 1069–1077, June 2010
34- B Westover, J Cormier, M Bianchi, M Shafi, R Kilbride, A
Cole, S Cash. Revising the ‘‘Rule of Three ’’ for inferring
seizure freedom ; Epilepsia, 53(2):368–376, 2012
PubMed
|