ARTÍCULOS ORIGINALES
Expression of
vegf-a, hif-1 α, cd34 and ki67 in clear cell renal cell
carcinomas and their relationship with conventional
prognostic markers
Bürgesser María Virginia*, Riva Verónica*, Ojeda Silvia
María**, Muñoz Morales Duberney***, Calafat Patricia*,
Diller Ana*
Revista Facultad de
Ciencias Medicas 2014; 71(1): 7-15
*Servicio de Patología,
Hospital Privado de Córdoba
**Doctora en Matemática – Facultad de Matemática, Astronomía
y Física – Universidad Nacional de Córdoba
***Licenciado en Matemáticas y Estadísticas – Facultad de
Ciencias Exactas, Físicas y Naturales – Universidad Nacional
de Córdoba
CORRESPONDING AUTHOR DATA: Bürgesser María Virginia.
Naciones Unidas 346. Parque Vélez Sarsfield. Córdoba,
Argentina X5016KEH
Teléfono: 54-351-4688829 Fax: 54-351-4688826. Email:
virburgesser@gmail.com
ACKNOWLEDGEMENTS: This study was supported by a grant from
the Florencio Fiorini Foundation and the Argentine Medical
Association.
CONFLICT OF INTERESTS: The authors declare that there is no
conflict of interests.
Introduction
In recent years attention has been focused on the expression
of different angiogenic factors in renal cell carcinomas,
especially in clear cell renal cell carcinomas (CCRCC)
because it is the most frequent variant of renal carcinoma (RCC),
accounting for 80% of the total. To date, tumor stage and
nuclear grade were considered the most important prognostic
variables for patients with CCRCC.
It is known that angiogenesis is an important factor that
enhances tumor growth, favoring invasion and dissemination.
Its development would respond to the biallelic loss of tumor
suppressor gene von Hippel Lindau (VHL) present in 50 to 70%
of sporadic CCRCC. This gene encodes a protein component of
the unit cell that breaks down various angiogenesis-inducing
factors such as hypoxia inducible factor 1α (HIF-1α). This
factor acts regulating the cellular stress in an hypoxic
microenvironment, determining the expression of growth
factors, including the vascular endothelial growth factor A
(VEGF-A). By altering the degradation of HIF-1α, its
persistence leads to increased expression of VEGF-A,
allowing increased tumor vascular development1,2,3. High
HIF-1α and VEGF-A levels have been correlated with high
vascular density, higher proliferation rate, higher nuclear
grade and advanced tumor stage, determining a poor clinical
outcome. Nevertheless, in RCC conflicting results have been
presented about protein levels of HIF-1α and VEGF-A,
vascular density and different clinicopathological factors.
The aim of the present study was to evaluate the
relationship between immunohistochemical expression of HIF-1α
VEGF-A, CD34 and Ki67 and clinicopathological parameters in
83 samples of CCRCC.
Material and Methods
Case selection, clinical data and population features
This study included tumor specimens of CCRCC obtained from
patients undergoing partial or total nephrectomy at Hospital
Privado, Córdoba, Argentina. 83 cases were selected from
filed formalin fixed and paraffin embedded tumor tissues
collected consecutively from January 2006 to December 2010.
All cases were reviewed by two pathologists using WHO (2009)
tumor classification criteria. Clinicopathologic data
obtained from patient medical records and from files kept at
Department of Pathology, included sex, age, tumor size,
pathological stage, presence of necrosis, nuclear grade as
assessed using Fuhrman nuclear grading system, vascular
invasion, capsular involvement, sinus tissue invasion,
urinary tract involvement and overall survival (OS).
Selection of tumor samples
Tumor samples were chosen using original hematoxilin/eosine
slides. All the slides from each case were carefully
evaluated to determine which the better block was,
considering the presence of enough viable tumor cells to
perform the evaluation of the four different
immunohistochemistry markers. Each sample corresponded to a
representative block of each case, avoiding those ones with
important areas of necrosis or fibrosis. The chosen block
was studied in full. In patients with metachronic
carcinomas, the larger tumor was studied.
Immunohistochemistry
The selected samples for immunohistochemistry were
deparafinated and rehydrated.
The process of antigenic recovery were carried out by using
a citrate buffer ph6 (DAKO) for HIF-1α and Ki67, EDTA buffer
ph9 (DAKO) for VEGF-A and pepsin for 20 minutes at 37ºC to
determine CD34.
The blockage of endogenous peroxidase was performed with
H2O2 at 0,3% in methanol for 30 minutes.
Primary antibodies used were HIF-1α, EP1215Y clone,
Millipore, in a dilution 1/200 for 45 minutes;, Ki67, Mib-1
clone, DAKO, in a dilution of 1/100 for 30 minutes; VEGF-A,
VG1 clone, DAKO, in a dilution of 1/50 for 60 minutes; and
CD34, QEnd10 clone, DAKO, in a dilution 1/50 for 30 minutes.
All antibodies were incubated at room temperature.
To reveal the technique, LSAB + kit HRP of DAKO were used
according to the manufacturer protocol (15 minutes of the
secondary antibody and 15 minutes of the tertiary reagent).
Diaminobencidine (DAB-DAKO) as chromogen was used for 10
minutes.
All samples were processed in an Autostainer Plus of DAKO
and they were counterstained with hematoxylin before their
visualization.
Evaluation of immunostaining
Immunohistochemical staining results were evaluated
independently by two pathologists, without knowledge of
clinicopathologic data on each individual case. Appropriate
positive and negative controls were included for each
antibody.
HIF-1α immunoreactivity was evaluated as percentage of
nuclear positivity. At least 10 high-power fields including
tumor were evaluated. Weak cytoplasmic staining was detected
in normal renal tubules and mesangial cells.
The immunostaining of VEGF-A was evaluated as percentage of
cytoplasmic staining pattern in tumor cells. At least 10
high-power fields including tumor were evaluated. Moderate
cytoplasmic staining was seen in normal renal tubules.
CD34 density was assessed by counting vessels of small
caliber in three high-power fields and calculating the mean
value.
Ki67 index was also quantified assigning a percentage value
that was calculated by scoring 500 tumor cell nuclei.
Illustrative pictures are shown in figure 1.
Figure 1
A: clear cell renal cell carcinoma (H/E – 10x)
B: clear cell renal cell carcinoma (H/E – 40x)
C: VEFG-A staining in clear cell renal cell carcinoma
(10x)
D: HIF-1α staining in clear cell renal cell carcinoma
(10x)
E: CD34 staining in clear cell renal cell carcinoma
(10x)
F: Ki67 staining in clear cell renal cell carcinoma
(10x)
Statistical analyses
All statistical analyses were done using the software IBM
SPSS Statistics version 15.0. Different statistical tests
were performed to assess the influence of angiogenic factors
(HIF-1α and VEFG-A), vascular density (CD34) and
proliferation factor (Ki67) among each other and among other
clinicopathological variables such as tumor size, Furhman
grade, capsular invasion, sinus tissue invasion, urinary
tract involvement, presence of necrosis and tumor stage
according TNM - UICC 2009. The linear association among
variables of continuous nature was assessed with Pearson´s
correlation. By means of studies of binary logistic
regression, variables associated to an increase or decrease
of the probability of events of interest were stated.
Kruskal Wallis test was applied as a non-parametric
alternative to the analysis of variance (ANOVA).
Overall survival was calculated from date of diagnosis to
date of death or last follow-up. Distribution of OS was
estimated using the method of Kaplan-Meier and differences
in OS was assessed by the stratified log-rank test.
Results
Clinical and pathological features
The study included 83 patients who had a diagnosis of CCRCC.
73.5% (61) were males and 26.5% (22) were females. The mean
age was 57 years (range 27 to 78 years); median age was 59
years.
26.5% (22) had a partial nephrectomy and 73.5% (61)
underwent total nephrectomy.
Regarding tumor size, the mean value was 49 mm (range 18 to
130 mm); median value was 50 mm.
Most cases presented with localized tumors (80%) (66). 64%
(53) were at pathological stage I, 16% (13), at stage II and
20% (17), at stage III.
According to Fuhrman nuclear grading system, 12% (10) tumors
were grade IV, 53% (44), grade III, 34% (28), grade II and
1% (1), grade I.
31% (26) showed necrosis above 10%.
Renal vein invasion was informed in 17% (14), capsular
invasion in 18% (15), sinus tissue invasion in 10% (8) and
urinary tract involvement in 7% (6) (table 1).
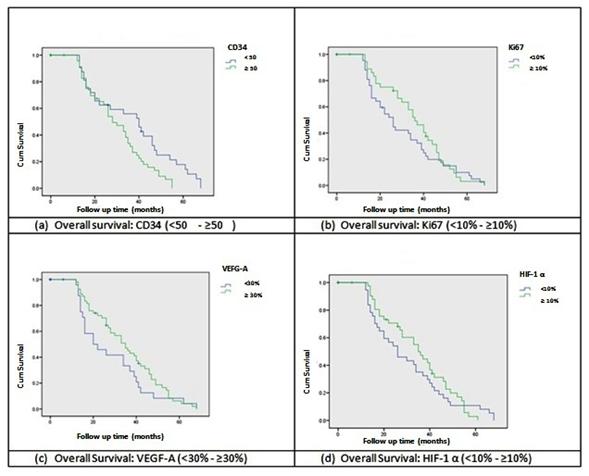 |
Figure 2
Kaplan-Meier survival analysis curves comparing groups
according immunohistochemical expression of the four
different markers
OS time was 24 months (range 6 to 68 months). Twelve
patients developed metastases in their follow-up, affecting
lung, bone and adrenal gland. Four of them died of the
disease. Three patients died immediately after surgery due
to hemorrhagic complications. At the end of this study, 84%
of the patients were alive.
Five patients had tumors with sarcomatoide differentiation
and three patients had metachronic bilateral carcinomas.
Statistical analisis
Pearson´s correlation stated that CD34 expression was
linearly associated with HIF-1α expression (p=0,029).
Likewise, HIF-1α expression was directly associated with
VEGF-A expression (p<0,000) and VEGF-A expression was
related to the proliferation index (Ki67) (p=0,010) (direct
linear association). Finally, OS was found in inverse linear
relationship with tumor size (p=0,006) and CD34 expression
(p=0,048).
Kruskal Wallis test found that CD34 expression was lower in
presence of necrosis (p=0,029), while Ki67 expression showed
statistically significant differences according to tumor
stage (p=0,006). Proliferation index was higher in T3 stage
regarding T1 and T2 stages (localized tumors).
In the studies of logistic regression, the following results
were found. Expression of HIF-1α was directly related to
Furhman grade (I-II vs. III-IV) (p=0.029), invasion of the
renal vein (p=0.016) and tumor stage (T1-T2 vs. T3)
(p=0.040). Likewise, tumor proliferation index, assessed
with Ki67, was directly related to the presence of necrosis
(p=0.008), capsular invasion (p=0.036) and advanced tumor
stage (p=0.007). Regarding expression of CD34, a higher
vascular density was inversely related to tumor necrosis
(p=0.031).
Tumor size turned out to be a factor which was constantly
related to features of locorregional aggressiveness such as
capsular invasion (p=0.018), invasion of the renal vein
(p=0.000), sinus tissue invasion (p=0.037), urinary tract
involvement (p=0.006), presence of necrosis (p=0.000) and
advanced tumor stage (p=0.000). Besides, it was found in
inverse relationship with OS.
OS regarding CD34 expression was lower in those patients
with vascular density equal or higher than 50 (Long-Rank of
Mantel-Cox, p=0.017). With respect to proliferation index,
OS did not turn out statistically different in patients with
an expression lower than 10% and higher or equal than 10%
(Long-Rank of Mantel-Cox, p=0.354). A similar result was
obtained for the survival analysis regarding VEGF-A factor,
comparing patients with an expression lower than 30% and
higher or equal than 30% (Long-Rank of Mantel-Cox, p=0.189).
There were no differences found in OS for patients with HIF-1α
expression under 10% compared to patients with HIF-1α
expression, higher or equal to 10% (Long-Rank of Mantel-Cox,
p=0.562). These results may be appreciated from the
observation of Kaplan-Meier curves on figure 2.
Discussion
The
last years, attention has been focused in the expression of
several angiogenic factors in RCC, especially in CCRCC since
they are the most frequent variety of renal carcinoma and
they would have more vascularization regarding the remaining
subtypes. It is known that angiogenesis is a favoring event
of tumor growth, although the mechanisms involved in its
development have not been elucidated clearly yet. Besides,
it plays an important role in tumor invasion and spread.
In this study, 83 cases of CCRCC processed in the Pathology
Department of Hospital Privado de Córdoba were reviewed so
as to interpret the expression of angiogenic factors,
vascular density and proliferation index in order to
correlate findings with regularly assessed
clinicopathological characteristics in this type of tumors.
According the different angiogenic factors studied in the
available literature, VEFG-A and HIF-1α have been the most
important ones, the results being controversial and
inconsistent according to different studies. Attention has
also been focused in tumor microvascular density, a feature
directly associated to the expression of angiogenic factors.
Proliferation index was also assessed with different results.
Concerning expression of VEGF-A, in 1997, Nicol et al showed
that it was increased in RCC, using Western Blot techniques
and immunohistochemistry4. In 1994, Takahashi et al,
evaluated the expression of mARN of VEGF-A in RCC and
detected an increase compared to normal renal parenchyma5
. Afterwards, two studies, (Tomisawa et al; Zhang et al)
showed that expression of VEGF-A was directly related to
tumor stage6,7. Besides, Zhang et al proposed that a
higher expression of VEGF-A was directly associated to a
higher vascular density7. Paradis et al showed that
expression of VEGF-A increased with higher tumor size and
was correlated to vascular density8. Jacobsen et al found
a direct relationship between VEGF-A expression and tumor
stage9. Yilmazer et al found that a higher expression of
VEGF-A was found in direct relationship with tumor size,
stage, vascular density and capsular invasion10. In 2009
Djorevic et al reported that VEGF-A expression increased
related to a higher nuclear grade, higher stage and higher
size. VEFG-A expression was not related to proliferation
index11. Even though, in 2007, the same group had
reported that a higher expression of VEGF-A was related to a
higher nuclear grade and a higher proliferation index12.
On the other hand, Minardi et al did not find any
association between VEGF-A expression and tumor stage,
nuclear grade, capsular invasion and necrosis. They did
report a direct relationship with HIF-1α expression and
vascular density13. In this study, the expression of VEGF-A
was only directly related to proliferation index. A
statistically significant relationship between the VEGF-A
expression and the remaining analyzed variables was not
found.
Regarding the expression of HIF-1α, Lidgren et al in 2006
did not find any association between HIF-1α expression with
tumor stage, nuclear grade, tumor size or invasion of the
renal vein14. Klatte et al did not find any significant
differences between the expression of this factor concerning
stage or nuclear grade as well. They report that the lower
expression of HIF-1α was associated to smaller tumors15.
On the other hand, Djorevic et al reported that the
expression of HIF-1α increased in tumors with higher nuclear
grade, higher stage and higher size11. In our study, the
expression of HIF-1α was directly related to nuclear grade,
tumor stage and invasion of the renal vein.
If we focus in the vascular density, Sharma et al found that
tumor vascular density was higher in tumors of advanced
stage16. Kavantzas et al showed that a higher vascular
density was related to a higher nuclear grade17. On the
other hand, Nativ el al showed that vascular density was
lower regarding a higher nuclear grade18. Herbst et al
found an inverse relationship between vascular density in
regards to nuclear grade and proliferation index19.
MacLennan et al did not find a significant association
between vascular density and stage or nuclear grade<20.
Yagasaki et al reported that vascular density was lower as
the tumor size increases21. In this study, vascular
density was inversely related to the presence of necrosis,
and in a direct relationship with HIF-1α expression.
About to proliferation rate, Onda et al found that the
expression of Ki67 was in direct relationship with tumor
stage22. Zhang et al showed a direct relationship with
stage and nuclear grade23. In this study, this index
showed an association with presence of necrosis, capsular
invasion and advanced tumor stage.
Concerning OS, Jacobsen et al reported that those renal
tumors with an expression of VEGF-A which was higher than
30% had less survival9. Minardi et al found similar
results13. Yildiz et al found that a highest expression
of VEGF-A and a high proliferation index were correlated to
a lower survival24. In another study, Lidgren et al
reported that the highest expression of HIF-1α was a
favorable prognostic factor25. On the other hand, Klatte
el al reported that patients whose tumors showed high
expression of this marker have less survival15. Regarding
vascular density, MacLennan et al did not find a
relationship between tumor vascular density and OS20. On
the other hand, Joo et al found that a higher vascular
density was related to a worse survival26. Rioux-Leclercq
el at showed that a higher proliferation rate was associated
to less survival27. In our study, OS was significantly
lower in those patients whose tumors showed a higher
vascular density, assessed by CD34 expression.
If we analyze the reasons which may explain the existence of
very controversial results regarding the expression of these
factors related to angiogenesis in RCC, the differences may
be due to the use of different antibody clones for
immunohistochemistry technique, to the intra and inter-observer
variability to evaluate immunohistochemical expression of
the different markers and to the absence of standardized
values to interpret these immunohistochemistry studies. What
we must highlight in our study, given certain technical
limitations, is the measurement of the vascular density
which was performed through staining with CD34 and
estimating the average of small vessel counts in fields of
great magnification in a conventional optical microscope16,17,28
.
Up to date, there are no other studies where the
interrelationship between the expression of VEGF-A, HIF-1α,
CD34 and Ki67 was assessed among each other and with other
clinicopathological variables.
Finally, when assessing the interrelationship between the
expression of the four markers evaluated in this study, we
found a linear relationship regarding the tumor expression
of HIF-1α and VEGF-A. Besides we observed that they were
related to a higher vascular density and a higher
proliferation index, which suggests a close relationship
between the expression of angiogenic factors by the tumor,
which would induce a higher formation of vascular channels
and a higher proliferation rate. In addition to, the results
showed that VEGF-A predicted proliferation, a reduction in
CD34 expression predicted necrosis, Ki67 expression
correlated with a higher stage of cancer as well as a
reduction in OS, and HIF-1α expression predicted a higher
grade and a higher stage of cancer but did not correlate
with OS.
As a conclusion, although the findings are controversial,
the expression of angiogenic factors such as the ones
studied, VEGF-A and HIF-1α, in CCRCC is a acknowledged fact
and opens a research scenery where the importance of
generating prospective and more standardized studies is
highlighted to specify the role of these angiogenic factors
in tumor evolution and to evaluate the ability to
standardize results that allow better diagnostic and
prognostic studies of these tumors.
References
1. X. Na, G. Wu, C.K. Ryan, S. R. Schoen, P.A. di'Santagnese,
E.M. Messing, Overproduction of vascular endothelial growth
factor related to von Hippel-Lindau tumor suppressor gene
mutations and hypoxia-inducible factor-1 alpha expression in
renal cell carcinomas. J Urol 170 (2003) 588-592.
PubMed
2. W.G. Jr Kaelin, The von Hippel-Lindau tumor suppressor
gene and kidney cancer. Clin Cancer Res 10 (2004)
6290S-6295S.
PubMed --
FullText
3. M.S. Wiesener, P.M. Münchenhagen, I. Berger, N.V. Morgan,
J. Roigas, A. Schwiertz, J.S. Jürgensen, G. Gruber, P.H.
Maxwell, S.A. Löning, U. Frei, E.R. Maher, H.J. Gröne, K.U.
Eckardt, Constitutive activation of hypoxia-inducible genes
related to overexpression of hypoxia-inducible factor-1alpha
in clear cell renal carcinomas. Cancer Res 61 (2001)
5215-5222.
PubMed
4. D. Nicol, S.I. Hii, M. Walsh, B. Tell, L. Thompson, C.
Kennett, D. Gotley, Vascular endothelial growth factor
expression is increased in renal cell carcinoma. J Urol 157
(1997) 1482-1486.
PubMed
5. A. Takahashi, H. Sasaki, S.J Kim, K.I. Tubisu, T. Kakizoe,
T. Tsukamoto, Y. Kumamoto, T. Sigimura, M. Terada, Markedly
increased amounts of messenger RNAs for vascular endothelial
growth factor and placenta growth factor in renal cell
carcinoma with angiogenesis. Cancer Res 54 (1994) 4233-4237.
PubMed
6. M. Tomisawa, T. Tokunaga, Y. Oshika, T. Tsuchida, Y.
Fukushima, H. Sato, H. Kijima, H. Yamazaki, Y. Ueyama, N.
Tamaoki, M. Nakamura, Expression pattern of vascular
endothelial growth factor isoform is closely correlated with
tumour stage and vascularisation in renal cell carcinoma.
Eur J Cancer 35 (1999) 133-137.
PubMed
7. X. Zhang, M. Yamashita, H. Uetsuki, Y. Kakehi,
Angiogenesis in renal cell carcinoma: Evaluation of
microvessel density, vascular endothelial growth factor and
matrix metalloproteinases. Int J Urol 9 (2002) 509-514.
PubMed
8. V. Paradis, N.B. Lagha, L. Zeimoura, P. Blanchet, P.
Eschwege, N. Ba, G. Benoît, A. Jardin, P. Bedossa,
Expression of vascular endothelial growth factor in renal
cell carcinomas. Virchows Arch 436 (2000) 351-356.
PubMed
9. J. Jacobsen, T. Grankvist, A. Rasmuson, A. Bergh, G.
Landberg, B. Ljungberg, Expression of vascular endothelial
growth factor protein in human renal cell carcinoma. BJU
International 93 (2004) 297-302.
PubMed
10. D. Yilmazer, U. Han, B. Onal, A comparison of the
vascular density of VEGF expression with microvascular
density determined with CD34 and CD31 staining and
conventional prognostic markers in renal cell carcinoma. Int
Urol Nephrol 39 (2007) 691-698.
PubMed
11. G. Djorevic, K. Matusan-Ilijas, E. Babarovic, I.
Hadzisejdie, M. Grahovac, B. Grahovac, N. Jonjie, Hypoxia
inducible factor-1α correlates with vascular endothelial
growth factor A and C indicating worse prognosis in clear
cell renal cell carcinoma. J Exp Clin Cancer Res 20 (2009)
28:40. doi: 10.1186/1756-9966-28-40.
PubMed
12. G. Djordjevic, V. Mozetic, D.V. Mozetic, V. Licul, K.M.
Ilijas, E. Mustac, R. Oguic, Z. Fuckar, N. Jonjic,
Prognostic significance of vascular endothelial growth
factor expression in clear cell renal cell carcinoma. Pathol
Res Pract 203 (2007) (Abstract) 99-106.
PubMed
13. D. Minardi, G. Lucarini, A. Filosa, G. Milanese, A.
Zizzi, R. Di Primio, R. Montironi, G. Muzzonigro, Prognostic
role of tumor necrosis, microvessel density, vascular
endothelial growth factor and hypoxia inducible
factor-1alpha in patients with clear cell renal carcinoma
after radical nephrectomy in a long term follow-up. Int J
Immunopathol Pharmacol 21 (2008) 447-455.
PubMed
14. A. Lidgren, Y. Hedberg, K. Grankvist, T. Rasmuson, A.
Bergh, B. Ljungberg, Hypoxia-inducible factor 1alpha
expression in renal cell carcinoma analyzed by tissue
microarray. Eur Urol 50 (2006) 1272-1277.
PubMed
15. T. Klatte, D.B. Seligson, S.B. Riggs, J.T. Leppert, M.K.
Berkman, M.D. Kleid, H. Yu, F.F. Kabbinavar, A.J. Pantuck,
A.S. Belldegrun, Hypoxia-inducible factor 1 alpha in clear
cell renal cell carcinoma. Clin Cancer Res 13 (2007)
7388-7393.
PubMed
16. S.G. Sharma, N. Aggarwal, S.D. Gupta, M.K. Singh, R.
Gupta, A.K. Dinda, Angiogenesis in renal cell carcinoma:
correlation of microvessel density and microvessel area with
other prognostic factors. Int Urol Nephrol 43 (2011)
125-129.
PubMed
17. N. Kavantzas, H. Paraskevakou, S. Tseleni-Balafouta, K.
Aroni, P. Athanassiades, G. Agrogiannis, E. Patsouris,
Association between microvessel density and histologic grade
in renal cell carcinomas. Pathol Oncol Res 13 (2007)
145-148.
PubMed
18. O. Nativ, E. Sabo, A. Reiss, M. Wald, S. Madjar, B.
Moskovitz, Clinical significance of tumor angiogenesis in
patients with localized renal cell carcinoma. Urology 51
(1998) 693-696.
PubMed
19. C. Herbst, H. Kosmehl, K.J. Stiller, A. Berndt, M.
Eiselt, J. Schubert, D. Katenkamp, Evaluation of microvessel
density by computerised image analysis in human renal cell
carcinoma. Correlation to pT category, nuclear grade,
proliferative activity and occurrence of metastasis. J
Cancer Res Clin Oncol 124 (1998) 141-147.
PubMed
20. G.T. MacLennan, D.G. Bostwick, Microvessel density in
renal cell carcinoma: lack of prognostic significance.
Urology 46 (1995) 27-30.
PubMed
21. H. Yagasaki, N. Kawata, Y. Takimoto, N. Nemoto,
Histopathological analysis of angiogenic factors in renal
cell carcinoma. Int J Urol 10 (2003) 220-227.
PubMed
22. H. Onda, M. Yasuda, A. Serizawa, R.Y. Osamura, N.
Kawamura, Clinical outcome in localized renal cell
carcinomas related to immunoexpression of proliferating cell
nuclear antigen, Ki-67 antigen, and tumor size. Oncol Rep 6
(1999) (Abstract) 1039-1043.
PubMed
23. X. Zhang, I. Takenaka, Cell proliferation and apoptosis
with BCL-2 expression in renal cell carcinoma. Urology 56
(2000) (Abstract) 510-515.
Abstract
24. E. Yildiz, G. Gokce, H. Kilicarslan, S. Ayan, O.F. Goze,
E.Y. Gultekin, Prognostic value of the expression of Ki-67,
CD44 and vascular endothelial growth factor, and microvessel
invasion, in renal cell carcinoma. BJU Int 93 (2004)
1087-1093.
PubMed
25. A. Lidgren, Y. Hedberg, K. Grankvist, T. Rasmuson, J.
Vasko, B. Ljungberg, The expression of hypoxia-inducible
factor 1alpha is a favorable independent prognostic factor
in renal cell carcinoma. Clin Cancer Res 11 (2005)
1129-1135.
PubMed
26. H.J. Joo, D.K. Oh, Y.S. Kim, K.B. Lee, S.J. Kim,
Increased expression of caveolin-1 and microvessel density
correlates with metastasis and poor prognosis in clear cell
renal cell carcinoma. BJU Int 93 (2004) 291-296.
PubMed
27. N. Rioux-Leclercq, J.I. Epstein, J.Y. Bansard, B. Turlin,
J.J. Patard, A. Manunta, T. Chan, M.P. Ramee, B. Lobel, J.P.
Moulinoux, Clinical significance of cell proliferation,
microvessel density, and CD44 adhesion molecule expression
in renal cell carcinoma. Hum Pathol 32 (2001) (Abstract)
1209-1215.
PubMed
28. H. Toge, T. Inagaki, Y. Kojimoto, T. Shinka, I. Hara,
Angiogenesis in renal cell carcinoma: the role of tumor-associated
macrophages. Int J Urol 16 (2009) 801-807.
PubMed
|